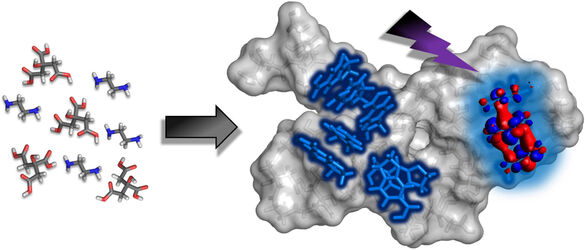
An international team of scientists, including experts from IT4Innovations, has revealed where excited states are localised in carbon dots and how accessible they are to the surrounding environment. Using supercomputer simulations and laboratory experiments, the researchers described the internal organisation of carbon dots, opening the way to their more efficient use in sensors and photocatalysis.
Carbon dots, discovered more than twenty years ago, are now considered among the most promising carbon nanomaterials thanks to their ability to emit light, biocompatibility, and ease of production. These properties have enabled their use in bioimaging, sensing, and optoelectronics. At the same time, they are being discussed as a potential sustainable alternative to conventional metal-semiconductor photocatalysts. Nevertheless, there has been no clear explanation of where exactly excited states form within these particles after illumination and how they interact with the surrounding environment – a question crucial for understanding the underlying mechanisms and for further development.
“We focused on non-carbonised carbon dots derived from citric acid and ethylenediamine. During their synthesis, fluorescent molecules known as IPCA form within the dots and, according to molecular dynamics simulations, spontaneously assemble into spatially separated domains rich in these fluorophores. A key finding is that these domains are not enclosed within the nanoparticle interior but are accessible to the solvent. As a result, excited electrons generated upon irradiation can directly react with molecules or ions from the surrounding environment, which is essential for both sensing and photocatalytic applications,” explains Michal Langer from IT4Innovations.
The research involved Michal Langer, Andrey L. Rogach, and Michal Otyepka from IT4Innovations, together with Lukáš Zdražil from VSB – Technical University of Ostrava and Silvio Osella from the University of Warsaw, and combined computer simulations with experiments. “Supercomputers at IT4Innovations played a key role in our research, enabling large-scale molecular simulations and quantum-chemical calculations. Subsequent laboratory experiments using luminescence quenching confirmed the accessibility of excited states to molecules from the surrounding environment,” adds Michal Langer.
This discovery provides a structural and mechanistic explanation of the reactivity of carbon dots with their surrounding environment. It offers concrete guidance for the targeted design and optimisation of these nanomaterials. In research published in the Carbon journal, the scientists not only explain the fundamental physicochemical mechanisms but also pave the way for nanomaterials with predictable behaviour, which is crucial for applications in energy, environmental science, and medicine.
Research article
Photoexcited species localize on solvent-accessible fluorophore-rich domains inside carbon dots
https://doi.org/10.1016/j.carbon.2026.121228
The research was supported by the REFRESH – Research Excellence For REgion Sustainability and High-tech Industries project (CZ.10.03.01/00/22_003/0000048) and Experimental and theoretical studies of near-infrared emitting chiral carbon dot luminophores project under the Global Experts programme (00734/2023/RRC).
Image*: Non-carbonized carbon dots derived from citric acid and ethylenediamine form dynamic assemblies containing fluorophore-rich IPCA domains. These domains act as distinct optical centers where photoexcited charges are generated and remain solvent-accessible, explaining both their efficient light emission and their capacity to interact with external species relevant to sensing and photocatalysis.
*Graphical abstract from: LANGER, Michal; ZDRAŽIL, Lukáš; ANDREY L. ROGACH; OSELLA, Silvio a OTYEPKA, Michal. Photoexcited species localize on solvent-accessible fluorophore-rich domains inside carbon dots. Online. Carbon. 2026, vol. 249, s. 121228-121228. ISSN 0008-6223.