Scientists from Charles University, IT4Innovations at the VSB – Technical University of Ostrava, and the Czech Academy of Sciences collaborate on research into the interaction of actinides with hydrogen. The results of their joint study on these elements have been published in the prestigious Reports on Progress in Physics journal.
The authors of the scientific article published in Reports on Progress in Physics journal – Ladislav Havela from Charles University, Dominik Legut from IT4Innovations, and Jindřich Kolorenč from the Czech Academy of Sciences – are researching the interaction of actinides with hydrogen and the physical properties of actinide hydrides. The interaction of actinides with hydrogen can lead to the breakdown of “components”. However, the affinity for hydride formation also has a positive side. Uranium metal is used to store and release tritium in nuclear fusion systems. The authors' main interest lies in understanding actinide metal hydrides and intermetallic compounds as solids combining the lightest and heaviest elements of the periodic table. The research article, published in the Reports on Progress in Physics journal, mentions the historical development since the 1940s and presents the results of their research, which elucidate the changes in electronic structure, crystal lattice properties, and spectroscopic characteristics due to the incorporation of hydrogen atoms in interstitial positions leading to volume expansion and charge transfers.
The published research answers questions about why uranium and plutonium hydrides are ferromagnetic. Magnetism in most uranium compounds occurs only at supercritical distances between the two nearest uranium atoms (the so-called Hill limit). However, this is not the case for the compound β-UH3 and its derivatives studied here, where electron transfer to hydrogen causes long-range exchange interactions and noncollinear ordering of ferromagnetism. Actinide compounds with hydrogen, e.g., LaH10, are also interesting because superconductivity has been predicted and experimentally verified at temperatures up to 260 K but at extremely high pressures, i.e., 180 GPa. The actual role of hydrogen and actinides in the mechanism of superconductivity is still under investigation.
Research article: https://doi.org/10.1088/1361-6633/acbe50
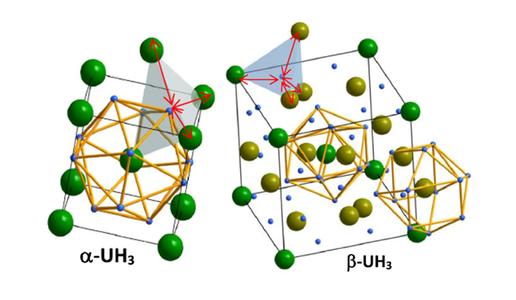
The interstitial positions of hydrogen atoms in the α-UH3 and β-UH3 structure (inside the uranium tetrahedron). The uranium atoms in β-UH3 are localized in Wyckoff positions 2a and 6c (distinguished by shades of green).